Descripción
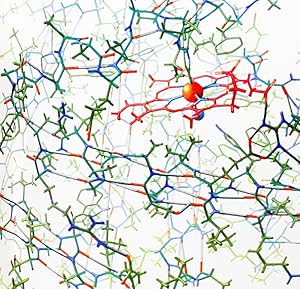
FIRST EDITIONS, OFFPRINT ISSUES (INCLUDING ONE SIGNED COPY AND ONE SIGNED PRESENTATION COPY), OF MILESTONE PAPERS ON THE DETERMINATION OF THE STRUCTURE AND FUNCTION OF PROTEINS, WORK FOR WHICH PAULING, KENDREW, AND PERUTZ ALL WON NOBEL PRIZES. Proteins - the "building blocks of life" - are themselves made up of building blocks known as amino acids. There are about twenty types of amino acid molecules commonly found in biological organisms, and each is chemically equipped to connect to two other amino acids through a linkage known as a peptide bond, enabling any number of amino acids to form a chain that constitutes the backbone of a protein molecule. (The specific linear sequence of amino acids that make up a particular protein's backbone is referred to as the protein's "primary structure.") Each of the 20-odd amino acids also has its own characteristic "side-chain." The interaction of these side chains with each other and with their natural environment in the cell determines how the protein will fold up after it is synthesized into a specific three-dimensional configuration that enables the protein to carry out its architectural, catalytic, or other functions. Some portions of the protein chain fold into simple, regular structural motifs such as helices and sheets. These are referred to as "secondary structure" of the protein, while the overall three-dimensional configuration of the entire protein molecule is known as its "tertiary structure." Because of the vital biological importance of proteins - and because the close relationship of between the biological function of a protein and its three-dimensional form - determining the details of protein structure has been one of the priorities of molecular biologists from the 1950s onward. The primary tool for unraveling the three-dimensional structure of proteins is X-ray crystallography, in which the complex diffraction patterns formed X-rays interacting with the lattice of molecules in a protein crystal can be decoded - through laborious mathematical calculations - in order to establish details of a protein's structure. As crystallographic techniques advanced and as faster and better computers became available, scientists were able to use this tool to determine the structure of proteins down to the atomic level. (The level of detail - or "resolution" - available from X-ray diffraction data is generally expressed in Ångstrom units [abbreviated Å]. One Å is one ten-millionth of a millimeter, or about the size of a hydrogen atom.) The papers that are offered here document key milestones in our understanding of protein structure. (a) A single inscribed offprint ("To Bob Schindler - Linus Pauling") containing eight papers by Linus Pauling and his collaborators, announcing the discovery of the "α-helix," the "Î -sheet," and other important aspects of the secondary structure of proteins, as published in the April and May 1951 issues of Proceedings of the National Academy of Sciences, including "The Structure of Proteins: Two Hydrogen-Bonded Helical Configurations of the Polypeptide Chain" and "The Pleated Sheet, a New Layer Configuration of Polypeptide Chains" (1st ed. 1951; offprint issue in original wrappers; boldly inscribed by Pauling to Robert Schindler). The offprint also includes a short related paper by Pauling and R. Corey, "Two Hydrogen-Bonded Spiral Configurations of the Polypeptide Chain", originally published in in 1950 in the Journal of the American Chemical Society. "In April of 1951, readers who turned the pages of PNAS were treated to a surprise .: seven studies from the same authors, published back-to-back, all on the subject of protein structure. . Grouping the papers together for maximum attention, authors Linus Pauling and Robert Corey must have realized the bombshell they had dropped on the scientific world. Knowledge of the inner workings of proteins - molecules often referred to as the building blocks of life - would be the key to understanding biology at the m. N° de ref. del artículo 2233
Contactar al vendedor
Denunciar este artículo
![]()